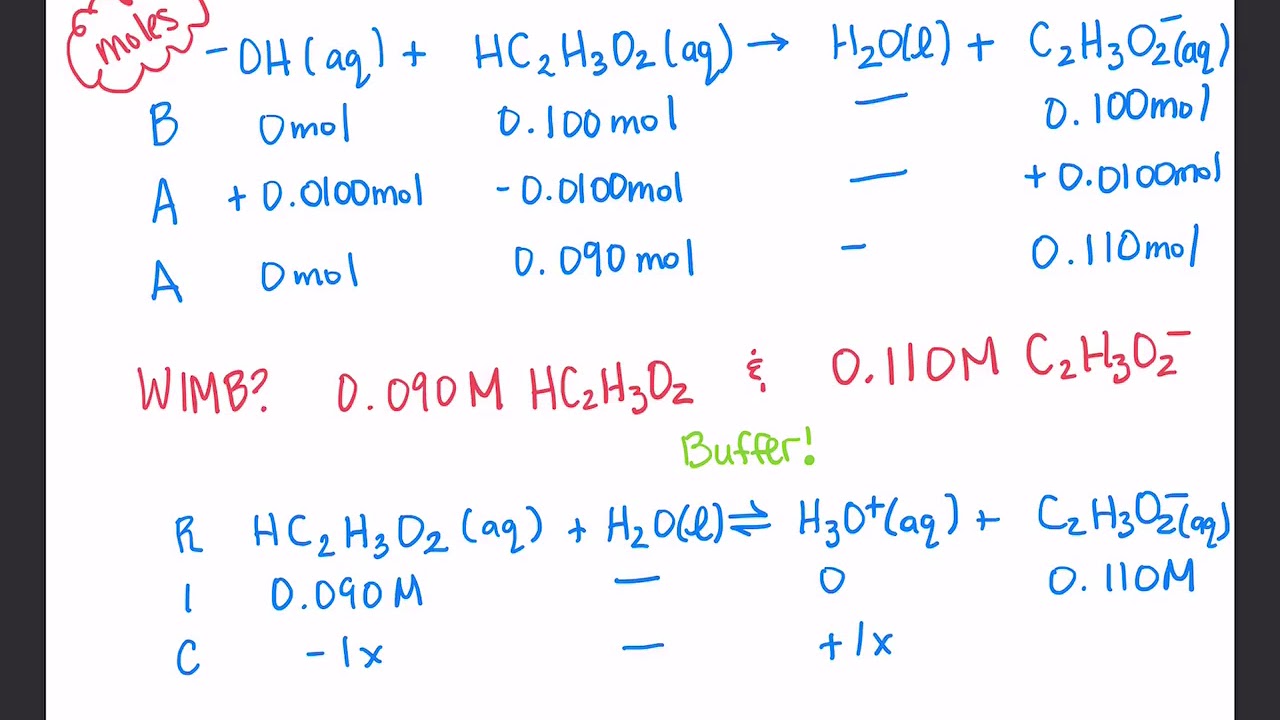
What Is The Purpose Of A Ph Buffer . It is able to neutralize. a buffer is an aqueous solution that has a highly stable ph. A buffering agent is a weak acid or. A solution whose ph is not altered to any great extent by the addition of small quantities of. what is buffer in chemistry? what is a ph buffer? A ph buffer is an aqueous solution consisting of a weak acid and its conjugate base or vice versa that. a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it.
from www.youtube.com
a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A solution whose ph is not altered to any great extent by the addition of small quantities of. what is buffer in chemistry? A buffering agent is a weak acid or. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. what is a ph buffer? A ph buffer is an aqueous solution consisting of a weak acid and its conjugate base or vice versa that. a buffer is an aqueous solution that has a highly stable ph. It is able to neutralize. a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of.
Calculating pH changes to a buffer solution YouTube
What Is The Purpose Of A Ph Buffer what is buffer in chemistry? what is a ph buffer? A solution whose ph is not altered to any great extent by the addition of small quantities of. It is able to neutralize. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. A ph buffer is an aqueous solution consisting of a weak acid and its conjugate base or vice versa that. a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of. what is buffer in chemistry? a buffer is an aqueous solution that has a highly stable ph. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffering agent is a weak acid or.
From www.youtube.com
14.10 Buffers Solutions that Resist pH Change YouTube What Is The Purpose Of A Ph Buffer what is buffer in chemistry? It is able to neutralize. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffering agent is a weak acid or. what is a ph buffer? a buffer is a solution that maintains the stability of a system’s ph level. What Is The Purpose Of A Ph Buffer.
From www.expii.com
Buffers — Definition & Overview Expii What Is The Purpose Of A Ph Buffer A buffering agent is a weak acid or. a buffer is an aqueous solution that has a highly stable ph. what is a ph buffer? It is able to neutralize. A ph buffer is an aqueous solution consisting of a weak acid and its conjugate base or vice versa that. A buffer solution is one which resists changes. What Is The Purpose Of A Ph Buffer.
From www.youtube.com
Calculating pH changes to a buffer solution YouTube What Is The Purpose Of A Ph Buffer what is a ph buffer? A buffering agent is a weak acid or. A ph buffer is an aqueous solution consisting of a weak acid and its conjugate base or vice versa that. A solution whose ph is not altered to any great extent by the addition of small quantities of. A buffer solution is one which resists changes. What Is The Purpose Of A Ph Buffer.
From uniglobalbusiness.com
pH Buffer Solution 7 Accurate pH Measurement HeavyDuty Gallon What Is The Purpose Of A Ph Buffer what is a ph buffer? a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffering agent is a weak acid or. a buffer is an aqueous solution that has a highly stable ph. what is buffer in chemistry? It is able to neutralize. A ph. What Is The Purpose Of A Ph Buffer.
From www.slideshare.net
Ph and buffer What Is The Purpose Of A Ph Buffer A ph buffer is an aqueous solution consisting of a weak acid and its conjugate base or vice versa that. what is buffer in chemistry? a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of. a buffer is an aqueous solution that has a highly stable ph. A. What Is The Purpose Of A Ph Buffer.
From www.slideserve.com
PPT Experiment 7 Preparation and Properties of Buffers PowerPoint What Is The Purpose Of A Ph Buffer It is able to neutralize. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. what is a ph buffer? a buffer is an aqueous. What Is The Purpose Of A Ph Buffer.
From www.youtube.com
Calculating the pH of a buffer YouTube What Is The Purpose Of A Ph Buffer It is able to neutralize. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffering agent is a weak acid or. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. what is buffer. What Is The Purpose Of A Ph Buffer.
From www.hopaxfc.com
Biological Buffers Products Hopax Fine Chemicals What Is The Purpose Of A Ph Buffer a buffer is an aqueous solution that has a highly stable ph. It is able to neutralize. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to. What Is The Purpose Of A Ph Buffer.
From www.youtube.com
pH of Buffer Solution (Example) YouTube What Is The Purpose Of A Ph Buffer A ph buffer is an aqueous solution consisting of a weak acid and its conjugate base or vice versa that. what is a ph buffer? A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. what is buffer in chemistry? a buffer is a. What Is The Purpose Of A Ph Buffer.
From www.mt.com
Technical buffer pH 4.01 250mL Bottle pH Buffer What Is The Purpose Of A Ph Buffer what is a ph buffer? a buffer is an aqueous solution that has a highly stable ph. a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of. It is able to neutralize. a buffer is a solution that can resist ph change upon the addition of an. What Is The Purpose Of A Ph Buffer.
From www.scribd.com
pH and Buffers Acid Buffer Solution What Is The Purpose Of A Ph Buffer what is a ph buffer? a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A ph buffer is an aqueous solution consisting of a weak acid and its conjugate base or vice versa that. A buffering agent is a weak acid or. what is buffer in chemistry?. What Is The Purpose Of A Ph Buffer.
From www.youtube.com
Buffers and pH Biology YouTube What Is The Purpose Of A Ph Buffer a buffer is an aqueous solution that has a highly stable ph. what is a ph buffer? A ph buffer is an aqueous solution consisting of a weak acid and its conjugate base or vice versa that. what is buffer in chemistry? a buffer is a solution that can resist ph change upon the addition of. What Is The Purpose Of A Ph Buffer.
From www.slideserve.com
PPT Chemical equilibria, principle of pH, buffers PowerPoint What Is The Purpose Of A Ph Buffer It is able to neutralize. A buffering agent is a weak acid or. A ph buffer is an aqueous solution consisting of a weak acid and its conjugate base or vice versa that. what is buffer in chemistry? A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added. What Is The Purpose Of A Ph Buffer.
From www.yokogawa.com
pH/ORP Buffer Calibration Solutions Yokogawa America What Is The Purpose Of A Ph Buffer It is able to neutralize. what is a ph buffer? a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. what is buffer in chemistry? A buffering agent is a weak acid or. A buffer solution is one which resists changes in ph when small quantities of an. What Is The Purpose Of A Ph Buffer.
From www.slideserve.com
PPT Chemical equilibria, principle of pH, buffers PowerPoint What Is The Purpose Of A Ph Buffer a buffer is an aqueous solution that has a highly stable ph. A solution whose ph is not altered to any great extent by the addition of small quantities of. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. a buffer is a solution that maintains the. What Is The Purpose Of A Ph Buffer.
From www.watts.com
pH Buffers HF scientific What Is The Purpose Of A Ph Buffer a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. A buffering agent is a weak acid or. It is able to neutralize. A solution whose ph. What Is The Purpose Of A Ph Buffer.
From www.instrumentchoice.com.au
3 Pack Of Ph Buffer Solution (Ph 4, Ph 7, Ph 10), 500Ml Each What Is The Purpose Of A Ph Buffer A solution whose ph is not altered to any great extent by the addition of small quantities of. A buffering agent is a weak acid or. a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of. a buffer is an aqueous solution that has a highly stable ph. Web. What Is The Purpose Of A Ph Buffer.
From www.studypool.com
SOLUTION Buffers and ph Studypool What Is The Purpose Of A Ph Buffer A solution whose ph is not altered to any great extent by the addition of small quantities of. It is able to neutralize. a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of. what is a ph buffer? a buffer is an aqueous solution that has a highly. What Is The Purpose Of A Ph Buffer.